Reactivity¶
Purpose¶
This benchmark assesses the MLIP’s capability to predict the energy of transition states (TS) and thereby the activation energy and enthalpy of formation of a reaction. Accurately modeling chemical reactions is an important use case to employ MLIPs to understand reactivity and to predict the outcomes of chemical reactions.
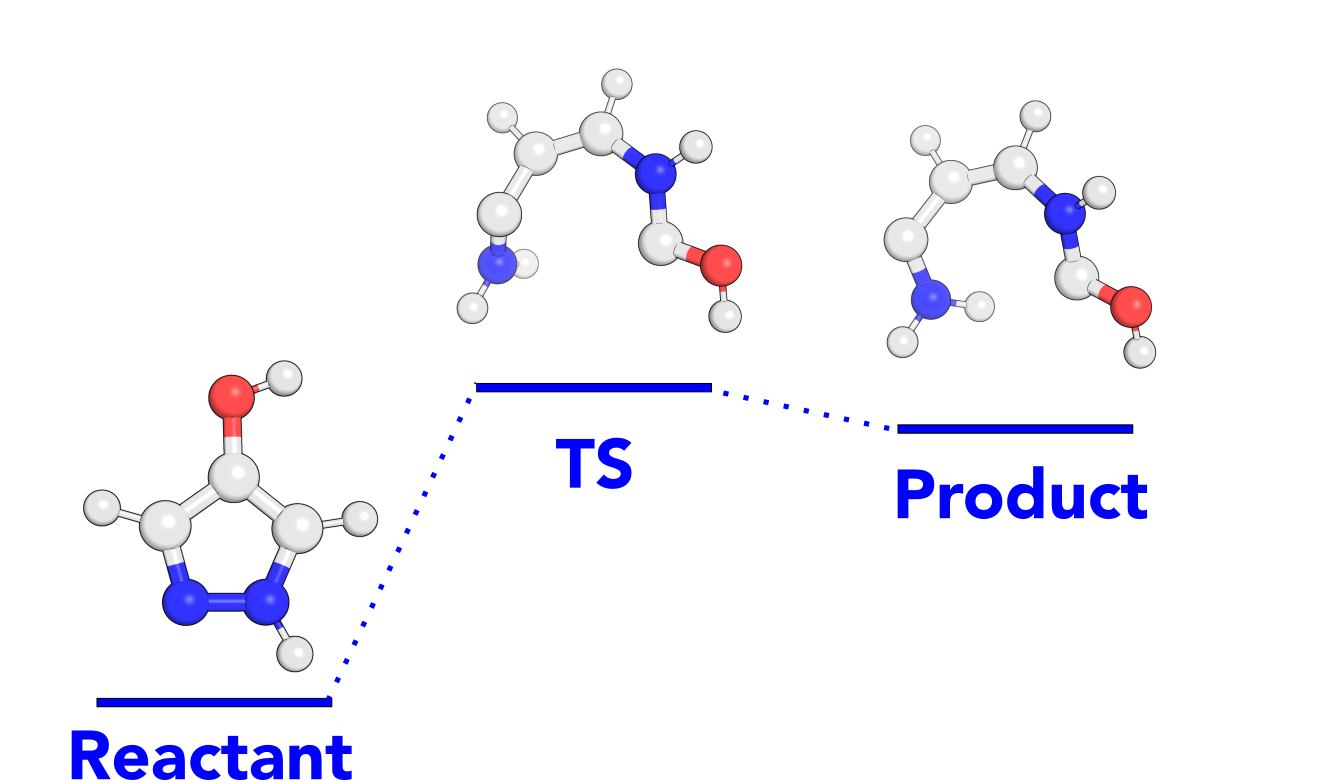
Chemical reaction example¶
Description¶
This benchmark leverages the mlip library for model inference, to predict the energy of reactants, products and transition states of a lare dataset of reactions. From the difference between these states, the activation energy and enthalpy of formation can be calculated. The performance is quantified using the MAE and RMSE in activation energy and enthalpy of formation.
Dataset¶
The dataset used for this benchmark is the Grambow [1] dataset which contains the reactants, products and transition states of 11960 reactions.
Interpretation¶
This benchmark tests the accuracy and ability to represent relative energy differences between the states of a reaction. Both, MAE and RMSE, should be as low as possible.